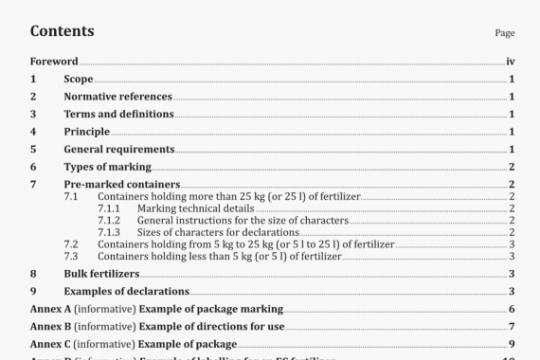
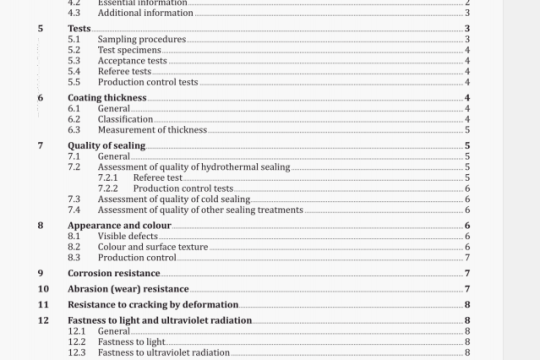
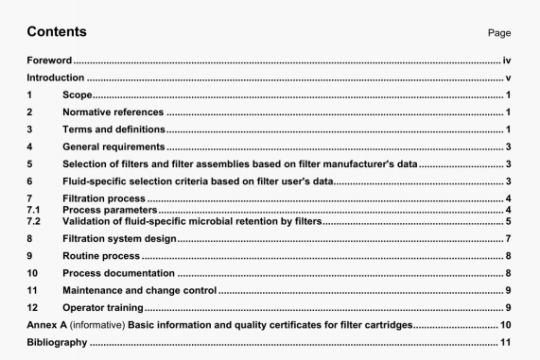
ISO 8044:2000 pdf download
ISO 8044:2000 pdf download.Corrosion of metals andalloys — Basic termsand definitions.
5.3 Double-sided adhesive tape
Ordinary commercially available 12-mm-wide tape is suitable.
5.4 Reference panel
A recommended reference panel for the calibration of abrasive paper is the PMMA panel, which is described in annex B.
Other reference panels may be used by agreement between the interested parties.
5.5 Balance
Use a balance with an accuracy of 0,1 mg.
6 Sampling
Take a representative sample of the product to be tested (or of each product in the case of a multi-coat system), as described in ISO 15528.
Examine and prepare each sample for testing, as described in ISO 1513.
7 Test panels
7.1 Substrate
The substrate shall be plane and, unless otherwise agreed, made of transparent glass, mild steel or aluminium in accordance with ISO 1514. However, paper sheeting which is suitable for the coatings under test may also be used.
3.13
bimetallic corrosion
contact corrosion (deprecated)
galvanic corrosion (3.12), where the electrodes (6.1.02) are formed by dissimilar metals
3.14
impressed current corrosion
electrochemical corrosion (3.01) due to the action of an external source of electric current
3.15
stray-current corrosion
impressed current corrosion (3.14) caused by current flowing through paths other than the intended circuits
3.16
pitting corrosion
localized corrosion (3.10) resulting in pits, I.e. cavities extending from the surface into the metal
3.17
crevice corrosion
localized corrosion (3.10) associated with, and taking place in, or immediately around, a narrow aperture or clearance formed between the metal surface and another surface (metallic or nonmetallic)
Licensed Copy: sheffieldun sheff
3.18
deposit corrosion
localized corrosion (3.10) associated with, and taking place under, or immediately around, a deposit of corrosion products (2.08) or other substance
3.19
water-line corrosion
corrosion (2.01) along, and as a consequence of the presence of, a gas/liquid boundary
3.20
selective corrosion
corrosion (2.01) of an alloy whereby the components react in proportions that differ from their proportions in the alloy
3.21
selective corrosion (3.20) of brass resulting in preferential removal of zinc
3.22
graphitic corrosion
selective corrosion (3.20) of grey cast iron, resulting in partial removal of metallic constituents, leaving graphite
3.23
intergranular corrosion
corrosion (2.01) in or adjacent to the grain boundaries of a metal
3.24
weld corrosion
corrosion (2.01) associated with the presence of a welded joint and taking place in the weld or its vicinity
3.25
knife-line corrosion
corrosion (2.01) resulting in a narrow slit in or adjacent to the filler/parent boundary of a welded or brazed joint
3.26
layer corrosion
corrosion (2.01) of internal layers of wrought metal, occasionally resulting in exfoliation, i.e. detachment of unattacked layers
Licensed Copy: sheffieldun sheff
NOTE Exfoliation is generally oriented in the direction of rolling, extrusion or principal deformation.
3.27
erosion corrosion
process invoMng conjoint corrosion (2.01) and erosion
NOTE Erosion corrosion can occur in, for example, pipes with high fluid flow velocity and pumps and pipe lines carrying fluid containing abrasive particles in suspension.
3.28
cavitation corrosion
process involving conjoint corrosion (2.01) and cavitation
NOTE Cavitation corrosion can occur, for example, in rotary pumps and on ships’ propellers.
3.29
fretting corrosion
process involving conjoint corrosion (2.01) and oscillatory slip between two vibrating surfaces in contact
NOTE Fretting corrosion can occur, for example, at mechanical joints in vibrating structures.
3.30
wear corrosion
process involving conjoint corrosion (2.01) and friction between two sliding surfaces in contact
3.31
corrosion fatigue
process involving conjoint corrosion (2.01) and alternating straining of the metal, often leading to cracking
NOTE Corrosion fatigue can occur when a metal Is subjected to cyclic straining in a corrosive environment (2.03).
3.32
stress corrosion
process involving conjoint corrosion (2.01) and straining of the metal due to applied or residual stress
Licensed Copy: sheffieldun sheffi
3.33
stress corrosion cracking
cracking due to stress corrosion (3.32)
3.34
hydrogen embrittlement
process resulting in a decrease of the toughness or ductility of a metal due to absorption of hydrogen
NOTE Hydrogen embrittlement often accompanies hydrogen formation, for example by corrosIon (2.01) or electrolysis, and can lead to cracking.
3.35
blistering
process resulting in dome-shaped defect visible on the surface of an object and arising from localized loss of cohesion below the surface
NOTE For example, blistering can occur on coated metal due to loss of adhesion between coating and substrate, caused by accumulation of products from localized corrosion (3.10). On uncoated metal, blistering can occur due to excessive Internal hydrogen pressure.