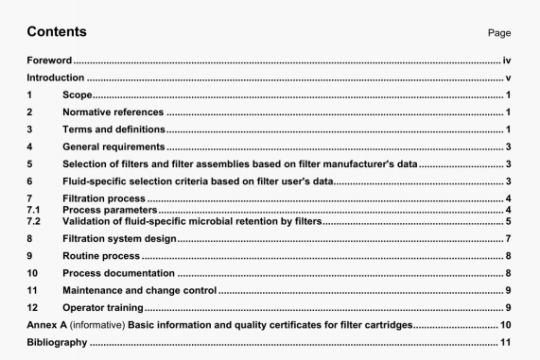
ISO 1431-3:2000 pdf download
ISO 1431-3:2000 pdf download.Rubber, vulcanized or thermoplastic一Resistance to ozone cracking — Part 3: Reference and alternative methods fordetermining the ozone concentration inlaboratory test chambers.
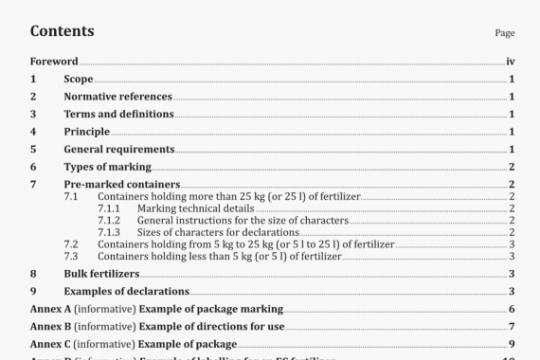
3 Principle
An ozone/air mixture is sampled from an ozone exposure chamber and the ozone concentration is determined by the UV absorption reference method or by alternative instrumental or chemical-analysis methods calibrated against the UV absorption method.
4 Apparatus
Apparatus used for the determination of the ozone concentration shall be one of the following types:
UV absorption
Electrochem ical
Chemiluminescence
Wet-chemical
The reference method is UV absorption, and all equipment shall be calibrated against the UV absorption method as specified in clause 5.
The apparatus used for the UV absorption method shall be in accordance with ISO 13964, except that it shall be capable of measuring ozone concentrations specified in ISO 1431-1 and -2.
Descriptions of alternative methods are given in annex B (instrumental methods) and annex C (wet-chemical methods).
5 Calibration
Calibration of the apparatus for determining the ozone concentration shall be in accordance with the procedures given in ISO 13964.
6 Procedure
The UV method shall be carried out in accordance with ISO 13964.
Other instrumental methods shall be used in accordance with the manufacturer’s instructions, attention being paid in particular to initial setting up, zero adjustment and maintaining and checking the instrument as mentioned in annex B.
Wet-chemical methods shall be carried out in accordance with annex C.
7 Expression of results
Generally, the ozone concentration is expressed in parts of ozone by volume per hundred million parts of air by volume (pphm).
However, the ozone concentration may also be expressed in mg/rn3 or in mPa. The expression mg/m3 indicates the number of ozone molecules in the volume which is available for ozone cracking and depends on both pressure and temperature.
For conversion purposes, the following equation is valid:
NOTE Under standard conditions of temperature (273 K) and pressure (1 atm.. 760 torr or 1 013 hPa), 1 pphm = 1,01 mPa
lt can be demonstrated that, for the same ozone content, by volume, of the ozonized air, measured at the samtemperature but at different atmospheric pressures, the partial pressure of ozone and the number of moles of ozonevary in the same ratio as the atmospheric pressure.
The results of an interlaboratory test programme conducted in North Americal8] prove the effect of ambierpressure on the cracking rate at a constant volumetric ozone content.
Therefore, the expression of the ozone concentration in laboratory test chambers on a volume per volume basisinappropriate where differences in atmospheric pressure are likely to exist.
The effect of these variations can be corrected for by working at a constant test chamber pressure or by varying thevolumetric ozone content of the ozonelair mixture in an inverse ratio to the atmospheric pressure. The effect canalso be overcome by expressing the ozone concentration as the partial pressure of the ozone in ozonized air.
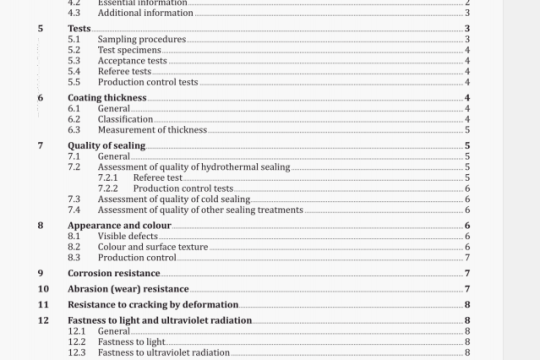
B.1.2 Apparatus
The analyser shall include a coulometric cell of the general type shown in Figure B.1. Standard models are available commercially.
The cathode is in the form of a platinum basket through which the ozonized air is bubbled. The anode can take the form of one of the following, although (b) is the preferred type:
a) a pool of mercury;
b) a silver mesh spiral.
The iodine liberated from the solution by the ozone is ionized at the cathode and is transported to the anode by the liquid circulation induced in the direction of the arrows by the bubbling action. At the anode, insoluble silver iodide or mercurous iodide is formed with the release of ionic charges which are exactly equivalent to the ozone introduced by the air stream.
The cell shall be connected to an analyser circuit of the general type shown in Figure B.2.
A stabilized d.c. voltage source is provided as a means of opposing the standard potential which appears at the cell terminals when ozone-free air is passed through the cell. This standard potential will depend on the anode material.
Thus, in the typical circuit shown in Figure B.2, the analyser can be calibrated directly by relating cell current toozone concentration.
B.2 Chemiluminescence
In chemiluminescence instruments, ozonized air is passed through a chamber where it comes into contact with a stream of ethylene, and the two gases undergo a chemiluminescence reaction with the emission of photons at about 430 nm. This emission of energy is measured by a photomultiplier and converted to an electrical output which isproportional to the ozone concentration.