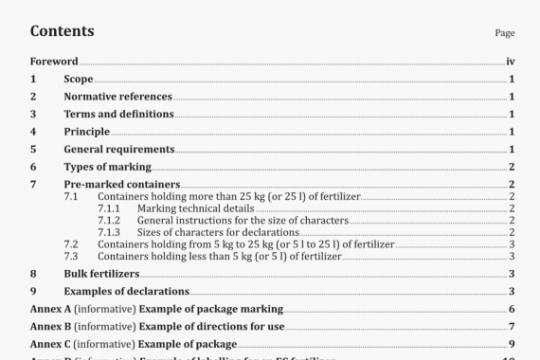
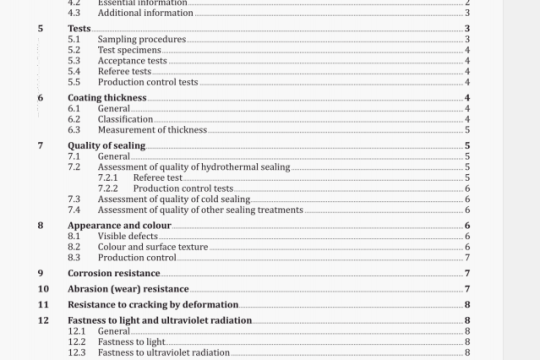
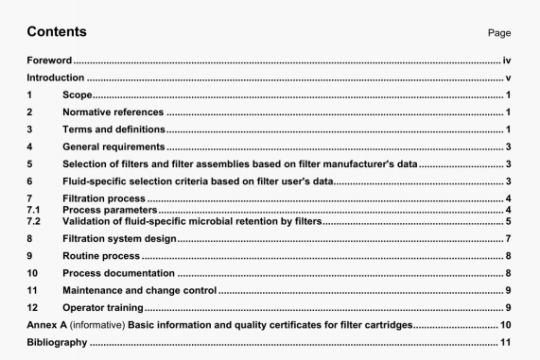
ISO 16017-1:2000 pdf download
ISO 16017-1:2000 pdf download.lndoor, ambient and workplace air —Sampling and analysis of volatile organiccompounds by sorbent tube/thermal desorption/capillary gas chromatography 一Part 1: Pumped sampling.
5.5 Standard atmospheres.
Prepare standard atmospheres of known concentrations of the compound(s) of interest by a recognized procedure. Methods described in Iso 6141, the appropriate part of ISO 6145 and ISO 6349 are suitable. If the procedure is not applied under conditions that allow the establishment of full traceability of the generated concentrations to primary standards of mass and/or volume, or if the chemical inertness of the generation system cannot be guaranteed, the concentrations shall be confirmed using an independent procedure.
5.6 Standard sorbent tubes, loaded by spiking from standard atmospheres.
Prepare loaded sorbent tubes by passing an accurately known volume of the calibration atmosphere through the sorbent tube, e.g. by means of a pump. The volume of atmosphere sampled shall not exceed the breakthrough volume of the analyte sorbent combination. After loading, disconnect and seal the tube. Prepare fresh standards with each batch of samples. Prepare standard atmospheres equivalent to 10 mg/rn3 and 100 jig/rn3. For workplace air, load sorbent tubes with 100 ml, 200 ml, 400 ml, 11, 2 I, or 4 I of the 10 mg/m3 atmosphere. For ambient or indoor air, load sorbent tubes with 100 ml, 200 ml, 400 ml, 11, 2 I, 4 I or 10 I of the 100 jIg/rn3 atmosphere.
5.7 Solutions for liquid spiking.
5.7.1 Solution containing approximately 10 mg/mI of each liquid component.
Accurately weigh approximately 1 g of substance or substances of interest into a 100 ml volumetric flask, starting with the least volatile substance. Make up to 100 ml with dilution solvent (5.2), stopper and shake to mix.
5.7.2 Solution containing approximately 1 mg/mI of liquid components.
Introduce 50 ml of dilution solvent into a 100 ml volumetric flask. Add 10 ml of solution 5.7.1 Make up to 100 ml with dilution solvent, stopper and shake to mix.
5.7.3 Solution containing approximately 100 jig/mI of each liquid component.
Accurately weigh approximately 10 mg of substance or substances of interest into a 100 ml volumetric flask, starting with the least volatile substance. Make up to 100 ml with dilution solvent (5.2), stopper and shake to mix.
5.7.4 Solution containing approximately 10 jig/mI of liquid components.
Introduce 50 ml of dilution solvent into a 100 ml volumetric flask. Add 10 ml of solution described in 5.7.3. Make up to 100 ml with dilution solvent, stopper and shake to mix.
5.7.5 Solution containing approximately 1 mg/mI of gas components.
For gases, e.g. ethylene oxide, a high level calibration solution may be prepared as follows. Obtain gas at atmospheric pressure by filling a small plastic gas bag from a gas cylinder containing pure gas. Fill a 1 -ml gas-tight syringe with 1 ml of the pure gas and close the valve of the syringe. Using a 2-mI septum vial, add 2 ml dilution solvent and close with the septum cap. Insert the tip of the syringe needle through the septum cap into the dilution solvent. Open the valve and withdraw the plunger slightly to allow the dilution solvent to enter the syringe. The action of the gas dissolving in the dilution solvent creates a vacuum, and the syringe fills with solvent. Return the solution to the flask. Flush the syringe twice with the solution and return the washings to the flask. Calculate the mass of gas added using the gas laws, i.e. 1 mole of gas at STP (standard temperature and pressure: 273,15 K and 1 013,25 hPa) occupies 22,4 litres, but correct for any non-ideality of the particular pure gas compound.
5.7.6 Solution containing approximately 10 pg/mI of gas components.
For gases, e.g. ethylene oxide, a low-level calibration solution may be prepared as follows. Obtain pure gas at atmospheric pressure by filling a small plastic gas bag from a gas cylinder. Fill a 1O-i.tl gas-tight syringe with 10 uI of the pure gas and close the valve of the syringe. Using a 2-mI septum vial, add 2 ml dilution solvent and close with the septum cap. Insert the tip of the syringe needle through the septum cap into the dilution solvent. Open the valve and withdraw the plunger slightly to allow the dilution solvent to enter the syringe. The action of the gas dissolving in the dilution solvent creates a vacuum, and the syringe fills with solvent. Return the solution to the flask. Flush the syringe twice with the solution and return the washings to the flask. Calculate the mass of gas added using the gas laws, i.e. 1 mole of gas at STP occupies 22,4 litres, but correct for any non-ideality of the particular pure gas compound.