ISO 16672:2003 pdf download
ISO 16672:2003 pdf download.Ophthalmic implants -Ocular endotamponades.
The intended performance shall be determined, taking into account published standards, published clinical and scientific literature, pre-clinical and clinical evaluation and clinical investigations.
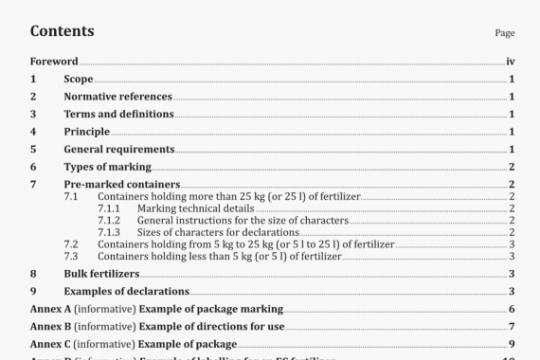
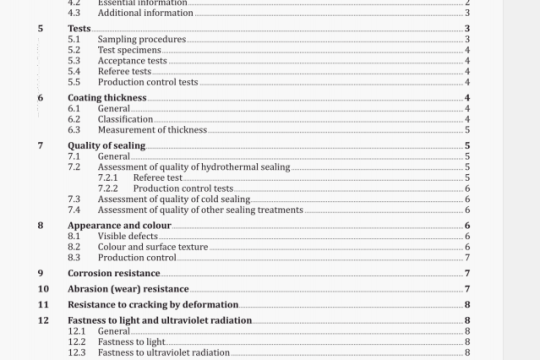
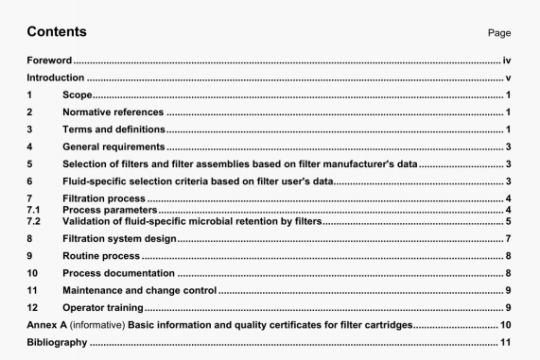
5 Design attributes
5.1 General
The general requirements for non-active surgical implants specified in ISO 14630 shall apply.
All testing requirements specified below shall be performed with finished, sterilized product, ready for release. Any analytical methods utilized shall be validated.
NOTE Tests described herein are intended to apply when qualifying materials and not necessarily as a routine quality assurance/control programme.
5.2 Chemical and biological contaminants
The identification of potentially hazardous chemical or biological contaminants shall be determined by a risk analysis. For raw materials of biological origin, these impurities may include proteins, nucleic acids or other biological materials. Contaminants of the finished product derived from the source materials or from the manufacturing process, such as cross-linking agents and antioxidants, that are potentially hazardous to the tissues of the eye, or systemically, shall be identified, whenever possible, and their concentration in the finished products reported.
Contaminants shall be determined using standard analytical methods when available, and all methods shall be described. Limits for identified contaminants shall be set and documented. Testing for the biological effects of these contaminants during evaluation of biological safety may be required if the risk analysis determines it necessary.
5.3 Chemical description
The manufacturer shall provide a description of each chemical component in the finished product and its quality specifications. If the component material is derived from biological sources, the organism from which it is obtained shall be stated along with its source. For synthetic polymers, the backbone and end-groups shall be identified. Residual monomers and reaction by-products shall be quantified and identified, if possible.
5.4 Concentration of the components
The concentration of each component material in the finished product shall be stated. Since the testing methodology may affect the actual concentration reported, the physical or chemical techniques utilized shall be described.
5.5 Density
The density of liquid forms of OEs shall be specified in kilograms per cubic metre (kg/rn3).
5.6 Gaseous expansion
For gaseous forms of OEs the intraocular gaseous expansion at (35 ± 2) °C and its dependence on atmospheric pressure shall be expressed.
5.7 Interfacial tension
Where applicable, the interfacial tension shall be expressed in newtons per metre (N/rn) at (35 ± 2) °C.
5.8 Kinematic viscosity
Where applicable, the kinematic viscosity shall be expressed in millimetres squared per second (mm2/s).
5.9 Molecular mass distribution
If the CE is a polymer, the average molecular mass and the polydispersity shall be reported.
The manufacturer shall conduct and report such additional tests as necessary to provide an adequate description of the molecular mass distribution of the components in the finished product. Whenever possible, standard methods shall be used and specified.
5.10 Particulates
An assessment of risk shall evaluate the potential for contamination by, or formation of, particulates in the product during manufacture, the conditions expected during transport and storage, and during use of the product and the associated hazards.
The manufacturer shall characterize and set limits for the types, range of sizes and levels of particles present in the finished product used in the clinical study. For each type of particle present, a limit which has been validated in a clinical study shall be set and an adequate justification for the limit shall be documented.
5.11 Refractive index
Where applicable, the refractive index between CE and air shall be measured with a refractometer at
(35 ± 2) °C and (546 ± 10) nm wavelength.
5.12 Spectral transmittance
The spectral transmittance of the CE shall be measured by transmission spectrophotometry over the range 300 nm to 1100 nm. Results shall be presented graphically, plotting percentage transmission against wavelength.
5.13 Surface tension
Where applicable, the surface tension shall be expressed in newtons per metre (N/rn) at (35 ± 2) °C.
5.14 Vapour pressure
Where applicable, the vapour pressure shall be expressed in conventional millimetres of mercury (mmHg) at (35 ± 2)°C.
6 Design evaluation
6.1 General.